Neisseria gonorrhoeae PCR
Summary
Nucleic acid amplification assay (PCR) for the detection of Neisseria gonorrhoeae DNA.
This is performed as a paired test with Chlamydia trachomatis PCR.
Usage
Diagnosis of Gonorrhoea – a common sexually transmitted infection; it is caused by the bacterium Neisseria gonorrhoeae.
Eye swabs can also be tested on neonates suspected of having opthalmia neonatorum.
How To Request On EPIC
For electronic requesting using EPIC (RDUH ONLY):
Search for: ‘Chlamydia trachomatis & Neisseria gonorrhoeae PCR‘ or test code ‘LAB1379‘
Specimen
Swabs – Endocervical /low vaginal/ rectal/ throat/urethral/ high vaginal swabs
- Self-taken female swabs are acceptable for screening purposes.
Urine (Preferably Males only – lower sensitivity than swabs for Females)
- First voided urine. The patient should not have passed urine in the preceding 1 -2 hours. If the specimen will not reach the laboratory on that day please store in the refrigerator.
Eye swabs (Please also send a bacterial swab for Neisseria gonorrhoeae.)
Specimen Container
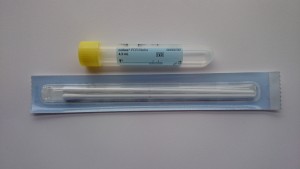
Yellow topped swabs (Cobas PCR)
White Top Universal Pots (20-30ml of initial stream urine)
Sample Collection
- Risk of invalid result due to lubricants
Products containing carbomer(s), including vaginal lubricants, creams and gels may interfere with the test and should not be used during or prior to collecting urogenital specimens.
-
- To minimize the risk of invalid test results:
- Refrain from using carbomer-containing products to lubricate a speculum as part of the collection procedure, or collect sample prior to use of lubricant/performing pelvic exam
- Ask patients to refrain from using feminine hygiene products for approximately 24 hours prior to their scheduled visit
- If patient is known to be using feminine hygiene products at time of collection, an alternative non-vaginal swab sample type may be considered
-
- Examples of carbomer-containing products include:
- DynaLube Lubricating Jelly
- McKesson Lubricating Jelly
- Cardinal Health Lubricating Jelly
- Medline Lubricating Jelly
- RepHresh Clean Balance
- RepHresh Vaginal Gel Prefilled
- Vagisil Anti-itch Cream
- Vagisil Crème Regular Strength
- Vagisil ProHydrate
- Vagisil Sensitive Cream
- IsoLove Balancing Gel
- Replens Long-Lasting Vaginal Moisturizer
- Metronidazole Vaginal Gel
- Endocervical and Low vaginal Swabs
- The specimen collection kit consists of a paper packet containing 2 swabs and a yellow topped plastic tube containing transport media.
-
- For an endocervical swab, it is essential to visualize the cervix. Any discharge or blood should be wiped away. Insert the swab into the cervix and rotate with the aim of removing epithelial cells.
-
- For taking a low vaginal swab, ensure the patient is not wearing a tampon. The labia should be parted to open the vagina. Insert a swab gently to about 5cm. Rotate all the way around the vagina
-
- If preferred the patient can take her own low vaginal swab ( instructions below)
-
- Please take only one sample per patient (vials with 2 swabs in will be rejected)
- How to collect a Low Vaginal Swab (self-swab)
1. Open the plastic bag. Open the paper packet which contains two swabs. Throw away the thin ended swab – you need to use the THICKER swab ONLY.
2. Stand with your feet apart and your knees bent, or sit down with your knees apart.
3. Take the thicker of the two swabs by the non-fluffy end. With your free hand hold open the skin around the vaginal opening.
4. Place the soft swab tip into the vagina approximately the length of your finger. Rotate the swab all the way around the vagina. Remove the swab and continue to hold it. Do not put it down.
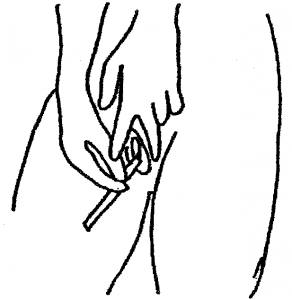
5. Holding the yellow topped tube upright, unscrew the cap and put the swab inside with the fluffy end down. There is a faintly scored ring around the white stick about 2/3 of the way up. Hold the swab stick firmly with the score mark against the top of the tube; bend it until it breaks off at the top of the tube. (There is liquid in the tube – DO NOT pour this away as it is meant to be there, without it the specimen cannot be processed.)
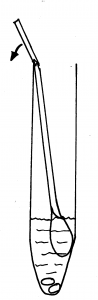
6. Screw the cap on firmly. Label the tube with your full name, date of birth and the date of the sample. Ensure that the tube only contains the thicker swab. The laboratory will not be able to process the sample if both swabs are in the tube.
7. You may discard the broken stick, packaging and unused swab into a household rubbish bag.
Special Instructions
- Please state time of collection and label with the minimum patient identification. Labelling must include the following:
- Surname
- First Name
- Date of Birth
- Unique Identifier – NHS Number (or Hospital No.)
- Samples from patients known to have blood transmissible viral infections should be clearly labelled “High Risk” or “Danger of Infection”.
- Please include relevant clinical details and any antimicrobial therapy the patient is taking
- If patient is symptomatic / high suspicion of infection please send charcoal swab for MC&S – Genital Swab Culture
Availability
Processed locally in Microbiology at RDUH
Turnaround Time
Please allow up to 6 days for result
Repeat Interval
Do not repeat this test within 1 month.
Method
Routine Testing: Roche Cobas 6800
Confirmatory & Urgent Testing: Cepheid GeneXpert
Useful Links
For Patients:
Gonorrhoea – Devon Sexual Health
Gonorrhoea – Lab Tests Online UK
Gonorrhoea – NHS
Gonorrhoea – Sexwise
For Healthcare professionals:
Gonorrhoea: guidance, data and analysis- UKHSA
British Association for Sexual Health and HIV
Specimen Labelling Procedure




